Practice Improvement Activity Options
Practice Improvement Modules
The ABOG Practice Improvement Modules are accessible via the dedicated ABOG portal, designed to support clinically active physicians in enhancing the quality of their medical practices. These modules serve as structured quality improvement exercises that guide physicians in identifying specific areas of their practice that can be enhanced. Diplomates have the option to choose from a list of available modules, each tailored to address an important aspect of obstetrics and gynecology. By engaging with these modules, physicians contribute to the overall advancement of patient care.
Diplomates selecting a module for their CC Part IV activity must read the module and answer the initial set of questions. Diplomates must also answer the follow-up set of questions available in their Physician Portal after 30 days.
There are two steps to complete a module:
1. Start.
Diplomates are to review the evidence-based module that describes characteristics and evidence for up to date, appropriate care. They then review up to ten of their patient charts, with attention to whether they adhered to the module’s evidence-based clinical approach.
2. Complete.
Thirty days later, Diplomates will receive an email from ABOG to complete a set of reflection questions regarding the module.
Requirements
- If you decide to complete a module, you must open and complete Phase 1 of that module each year during the six-year CC cycle. Phase 2 will be accessible one month after Phase 1 is completed.
- All modules must be completed by the end of Year 6 in the CC program.
Quality Improvement Efforts
ABOG will consider structured quality improvement (QI) projects in obstetrics and gynecology for Continuing Certification (“CC”) Part IV credit. These projects must demonstrate improvement in care and be based on accepted improvements in science and methodology.
Application Process for QI Efforts:
- Apply.
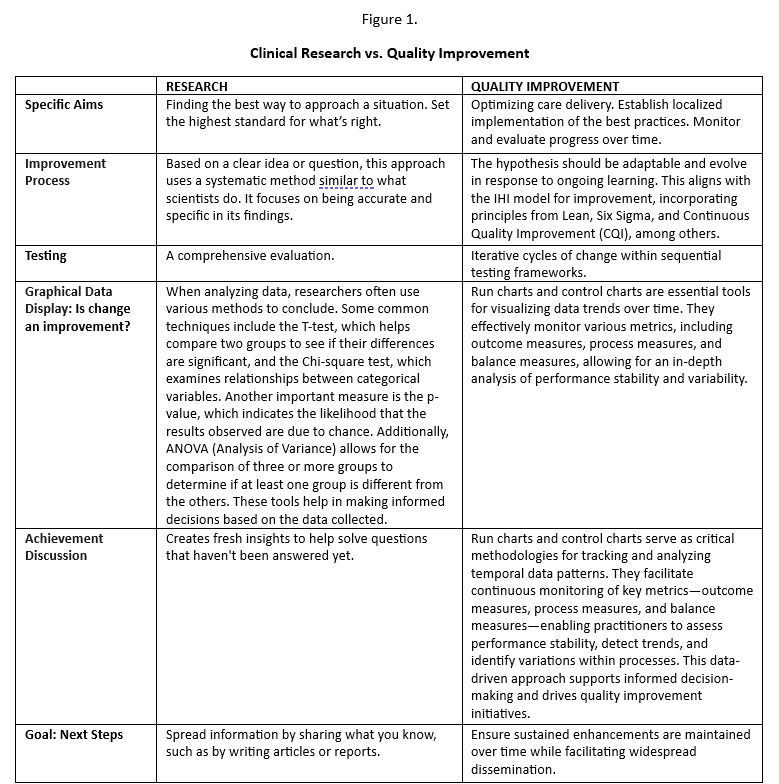
To apply, please submit your application to the CC Department by November 15, 2025. You can access the online Program Portfolio application system through the Part IV Practice Improvement section of the Physician Portal. Further details regarding application requirements are outlined below. (Fig 1)
- Review Process.
The ABOG staff reviews applications within a two-week timeframe. During this review period, applicants may be contacted for further clarification if needed.
- Report Participants.
The applicant must submit a list of participants by November 1, 2025. This is essential to ensure that processing is completed before the CC deadline on November 15, 2025. Participants must be entered through the online application system.
QI efforts in obstetrics and gynecology that qualify for CC Part IV credit must meet the following standards:
- Ensure that leadership and management at the project level are capable of overseeing adherence to participation criteria. This includes tracking participant details such as their dates of involvement and their roles in relation to the definition of meaningful participation.
- Address aspects of care that the physician can influence within one or more of the six Institute of Medicine quality dimensions: safety, effectiveness, timeliness, equity, efficiency, and patient-centeredness.
- Align measures with the Plan-Do-Study-Act (PDSA) Cycle.
- Establish a specific, measurable, specialty-relevant, and timely aim for improvement.
- Utilize appropriate, relevant, and evidence-based performance measures, including those related to patient care at the appropriate level of analysis (physician, clinic, care team, etc.).
- Incorporate suitable interventions to be tested for improvement.
- Implement thorough and consistent data collection and reporting of performance data to effectively assess the impact of the interventions over two or more improvement cycles.
- Represent an effort to translate or implement an improvement into routine care, or to disseminate or spread an existing improvement into practice.
- Ensure that there are sufficient and appropriate resources available to support the successful completion of the activity, while avoiding any conflicts of interest.
To earn CC Part IV credit for participating in approved QI efforts, physicians must:
- Attest that they have meaningfully participated in the approved QI effort,
- Have their attestation cosigned or reported to ABOG by the project leader; and
- Reflect on the QI effort.
Physician participation in an approved QI effort is considered meaningful when:
- The QI effort is intended to provide clear benefit to the physician’s patients and is directly related to the physician’s clinical practice and/or the specialty of Obstetrics and Gynecology.
- The physician is actively involved in the QI effort, including, at a minimum, working with care team members to plan and implement interventions, interpreting performance data to assess the impact of the interventions, and making appropriate course corrections in the improvement effort.
- The physician can personally reflect on the activity, describing the changes that were performed in their practice and/or institution, and how it affected the way the care is delivered.
Physicians can claim CC Part IV credit each time they meet meaningful participation requirements as long as they are implementing new interventions.
ABMS Portfolio Program
Many physicians engage in quality improvement (QI) within their practice and/or institution. The ABMS Portfolio Program offers healthcare organizations a way to approve these QI efforts for ABOG Continuing Certification (“CC”) Part IV credit.
Most Portfolio Program QI activities are institution or hospital-sponsored and can be multi-disciplinary or specific to Obstetrics and Gynecology. Projects must meet Portfolio Program standards and have ABOG approval. Long-term projects may be extended for ongoing QI activities. Diplomates who participate meaningfully in these projects will fulfill Part IV requirements for the 2025 CC.
To learn more about the ABMS Portfolio Program, visit their website .
Simulation Courses
ABOG recognizes simulation training as an approach to enhance a physician’s technical, clinical, and teamwork skills. Diplomates may participate in CME that involves simulation activities, provided that the CME is approved in advance by the Continuing Certification (“CC”) Department of ABOG. After reviewing the CME content and simulation activity, ABOG will approve CME that meets CC standards (has relevant and meaningful simulation and self-assessment).
The simulation activity must provide advanced, hands-on clinical education experiences for participants. It may integrate task-trainers, low- and high-fidelity simulators, computer-based simulations, and/or actual medical devices.
Application Process for Simulation Course
- Apply.
Apply before November 15, 2025, via the online Portfolio Program application system, MOCAM.
- Diplomates can access via the Part IV section of the ABOG Physician Portal.
- External Sponsors can apply to MOCAM by creating a Login and Password.
- Review Process.
ABOG staff will review the application within two weeks. During the review period, applicants may be asked for clarification.
- Report on Participants.
The applicant and/or sponsor representative will be responsible for submitting a list of participants by November 1st, 2025. This is essential to ensure that processing is completed before the CC deadline on November 15, 2025. Participants should be submitted within the MOCAM online application system.
Simulation Course Standards
To ensure high-quality learning experiences that meet the simulation requirements of CC, ABOG has established standards and will approve activities and CME courses that meet those standards. The following core curricular components for simulation courses must be present:
- A minimum of 4 (four) hours of total course instruction,
- Active participation in simulation procedures or scenarios,
- Feedback or post-scenario debriefing,
- One instructor must be an ABOG Diplomate in good standing,
- Sufficient and appropriate resources to support the successful conclusion of the activity without introducing a conflict of interest relevant to equipment or pharmaceutical manufacture or other commercial products that may bias the participant in their future practice,
- The instructor-to-student ratio must be no greater than 1:5.
Continuing Certification and CME Credit
To receive CC Part IV credit, the Diplomate must actively participate in the entire simulation course and complete a course evaluation. After the activity, the physician will receive email instructions to reflect on the simulation and answer web-based questions about the impact on their practice.
ABOG CC Part IV credit is independent of CME credit. Some activities and courses may provide CME credit. Diplomates should contact the site or sponsor for specific CME information about their courses.
Quality Improvement Publications
ABOG awards Continuing Certification (“CC”) Part IV credit for authorship and co-authorship of:
- Published articles relating to QI activities in healthcare. To be considered for CC Part IV credit, articles must be published in a peer-reviewed journal,
- Adhere to SQUIRE guidelines for published QI articles,
- Be published during the Diplomate’s current CC year.
Quality Improvement Presentations and Posters
ABOG also recognizes authorship and co-authorship of peer-reviewed oral presentations and posters presented at national scientific meetings that describe the implementation and outcomes of a QI project. The project's ultimate success will not affect the CC Part IV credit, but it should address a recognized gap in care, generally be prospective, and involve more than one QI cycle. To be considered for CC Part IV credit, abstracts or posters must include:
- The specific aim of the QI project. (Fig 1)
- The process for improvement.
- The progress toward or results of achieving the specific aim.
- A discussion of whether the aim was achieved, factors that affected success, and the next steps.
Process for QI Publications, Presentations, and Posters
- Apply.
Complete the Attestation Form accessible via the dedicated ABOG portal before November 15, 2025. The applicant must include a copy of the QI publication, presentation, or poster being submitted for consideration, and any other documents required.
- Review Process.
ABOG staff will review the documents within two weeks. During the review period, applicants may be asked for clarification.
- Credit for QI publications, posters, and presentations.
Applicants will receive Part IV credit upon approval; co-authors may also request credit if the criteria have been met.
Clinical Research
Clinical research, although important for advancing healthcare, does not qualify for Continuing Certification (CC) Part IV credit. (Fig 1)
Not Clinically Active
Diplomates occupying non-clinical roles, such as researchers with no clinical practice and those on sabbaticals, have the opportunity to maintain their certification by completing Continuing Certification (“CC”) Parts I, II, III, and IV. Diplomates must notify the American Board of Obstetrics and Gynecology (ABOG) of their current status and obtain approval for any requests for exemption from Part IV Practice Improvement requirements.
Application Process for Part IV Exemption:
- Apply.
Complete the Attestation Form accessible via the dedicated ABOG portal before November 15, 2025. Diplomates can access the document via the Part IV section of the ABOG Physician Portal.
- Review Process.
ABOG staff will review the application within two weeks. During the review period, applicants may be asked for clarification.
- Notification.
Once the review process has been completed, the Diplomate will be notified by email.
If a Diplomate is granted exemption from Part IV, they will retain their ABOG certification status, even though they are not actively engaged in clinical practice. Should these individuals decide to return to a clinical setting, they may contact ABOG to adjust their certification designation and resume Part IV activities.
Diplomates who have received exemptions from Part IV are still obligated to fulfill all other CC requirements by the deadline of November 15, 2025. This includes ensuring that all components of Parts I, II, and III are completed within the designated timeframes, thereby demonstrating a continued commitment to professional development and adherence to the standards of practice in the field of Obstetrics and Gynecology.
For additional information, please contact the Continuing Certification Department at continuingcert@abog.org.